Understanding Supercritical Water
Water – that is not liquid, gas, or solid – is the power behind Plantrose.
Plantrose technology uses water to break down biomass and convert it into its constituent parts for use in innovative materials for decarbonization and sustainability in an array of product applications in multiple industries.
In the Plantrose process, water acts as both a solvent and a catalyst, decrystalizing and dissolving the cellulose and hydrolyzing the naturally occurring polymers of the plant. The water is able to do this because it is reacting under supercritical conditions – as supercritical water (water under pressure at a high temperature).
Watch a supercritical reaction occur in slow motion to help visualize how particles are deconstructed during Plantrose conversion.
What is supercritical water?
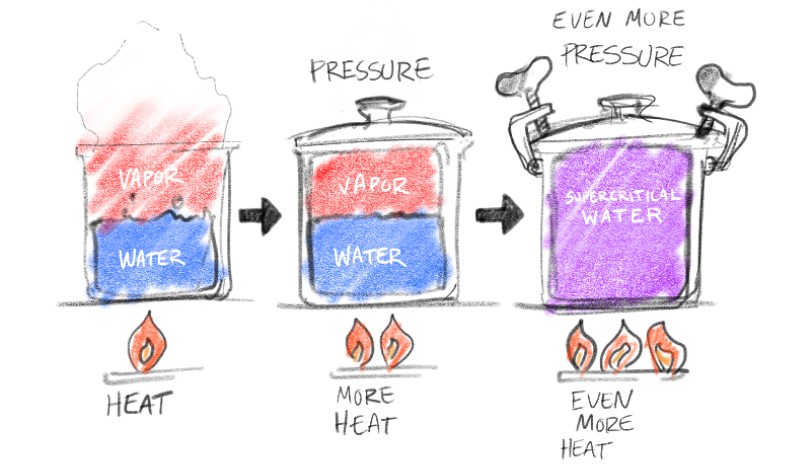
To understand supercritical water, you have to envision what happens to regular water when hot temperatures & high pressures are applied. At 373°C and 220 bars, normal water becomes supercritical water. "Supercritical" can be thought of as the "fourth state" of a material. It is not a solid, a liquid or a gas -- and appears as something like a vapor.
So, to picture supercritical water, think about a familiar example: boiling water on the stove. When a pot of water starts to boil, you can put pressure on the water and stop it from boiling; this occurs when we put a lid on that pot (or in a more extreme example, what happens in a pressure cooker). That action adds pressure — so the water will actually reach a higher temperature and go above the normal boiling point — until you get past a certain higher temperature, and then, suddenly it'll start boiling again.
Now imagine if you are in a laboratory and have the equipment to put even more pressure on the water, and it will again stop boiling. You can continue to increase temperature, and then pressure, and temperature until you get to about 373 degrees Celsius. At that time, when you reach the critical point, you can not compress the water back into being liquid - it always stays in something that looks like a vapor form. It’s completely compressible (as opposed to water in a liquid phase, that is not). That’s where water becomes supercritical.
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be 'fine-tuned'.
Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes. Carbon dioxide and water are the most commonly used supercritical fluids, being used for applications like decaffeination in coffee and nuclear power generation, respectively.
— Székely, "What is a supercritical fluid?", Budapest University of Technology and Economics
